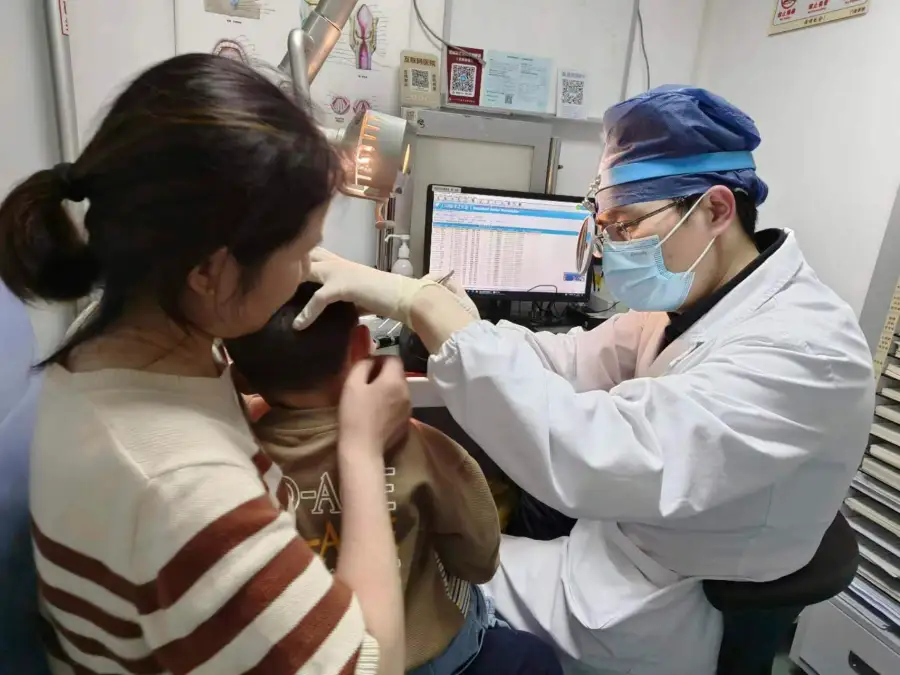
In a groundbreaking study published on Wednesday, researchers have successfully restored hearing in children with hereditary deafness using a type of gene therapy. The study, co-led by experts from Mass Eye and Ear in Boston, focused on six children with a genetic form of deafness known as DFNB9.
DFNB9 is caused by mutations in the OTOF gene, which results in the absence of a crucial protein called otoferlin. This protein is essential for transmitting sound signals from the ear to the brain.
The clinical trial, initiated in December 2022 at the Eye & ENT Hospital of Fudan University in Shanghai, involved administering an inactive virus carrying a functional version of the OTOF gene directly into the inner ear of the six children at varying doses. Over a period of 26 weeks, the children were closely monitored.
The results, published in The Lancet, revealed that five out of the six children, who were previously classified as having total deafness, regained their hearing and were able to conduct normal conversations. This marks the first human clinical trial utilizing gene therapy to treat this condition, with the largest number of patients treated and the longest follow-up period.
Dr. Zheng-Yi Chen, an associate scientist at Mass Eye and Ear, expressed excitement about the milestone, emphasizing the significant impact this breakthrough could have on the field of hearing loss treatment. He highlighted the absence of FDA-approved drugs for treating any type of hearing loss prior to this study.
Chen stressed the importance of early detection and intervention for children with hearing impairment, citing its crucial role in their overall development. He emphasized the need for expanded studies involving more patients over a longer duration to validate the effectiveness of the treatment and potentially extend its application to other genetic forms of deafness.
The findings from the clinical trial will be presented at the Association for Research in Otolaryngology Annual Meeting on February 3 in Anaheim, California. This achievement follows the recent report of the successful gene therapy treatment of an 11-year-old boy from Spain, marking significant progress in the field of genetic hearing loss reversal.
In a groundbreaking study published on Wednesday, researchers have successfully restored hearing in children with hereditary deafness using a type of gene therapy. The study, co-led by experts from Mass Eye and Ear in Boston, focused on six children with a genetic form of deafness known as DFNB9.
DFNB9 is caused by mutations in the OTOF gene, which results in the absence of a crucial protein called otoferlin. This protein is essential for transmitting sound signals from the ear to the brain.
The clinical trial, initiated in December 2022 at the Eye & ENT Hospital of Fudan University in Shanghai, involved administering an inactive virus carrying a functional version of the OTOF gene directly into the inner ear of the six children at varying doses. Over a period of 26 weeks, the children were closely monitored.
The results, published in The Lancet, revealed that five out of the six children, who were previously classified as having total deafness, regained their hearing and were able to conduct normal conversations. This marks the first human clinical trial utilizing gene therapy to treat this condition, with the largest number of patients treated and the longest follow-up period.
Dr. Zheng-Yi Chen, an associate scientist at Mass Eye and Ear, expressed excitement about the milestone, emphasizing the significant impact this breakthrough could have on the field of hearing loss treatment. He highlighted the absence of FDA-approved drugs for treating any type of hearing loss prior to this study.
Chen stressed the importance of early detection and intervention for children with hearing impairment, citing its crucial role in their overall development. He emphasized the need for expanded studies involving more patients over a longer duration to validate the effectiveness of the treatment and potentially extend its application to other genetic forms of deafness.
The findings from the clinical trial will be presented at the Association for Research in Otolaryngology Annual Meeting on February 3 in Anaheim, California. This achievement follows the recent report of the successful gene therapy treatment of an 11-year-old boy from Spain, marking significant progress in the field of genetic hearing loss reversal.